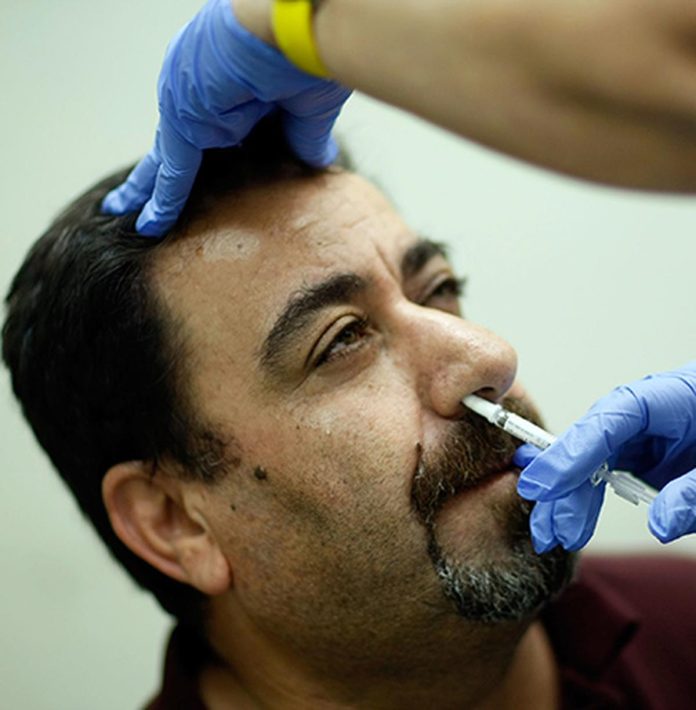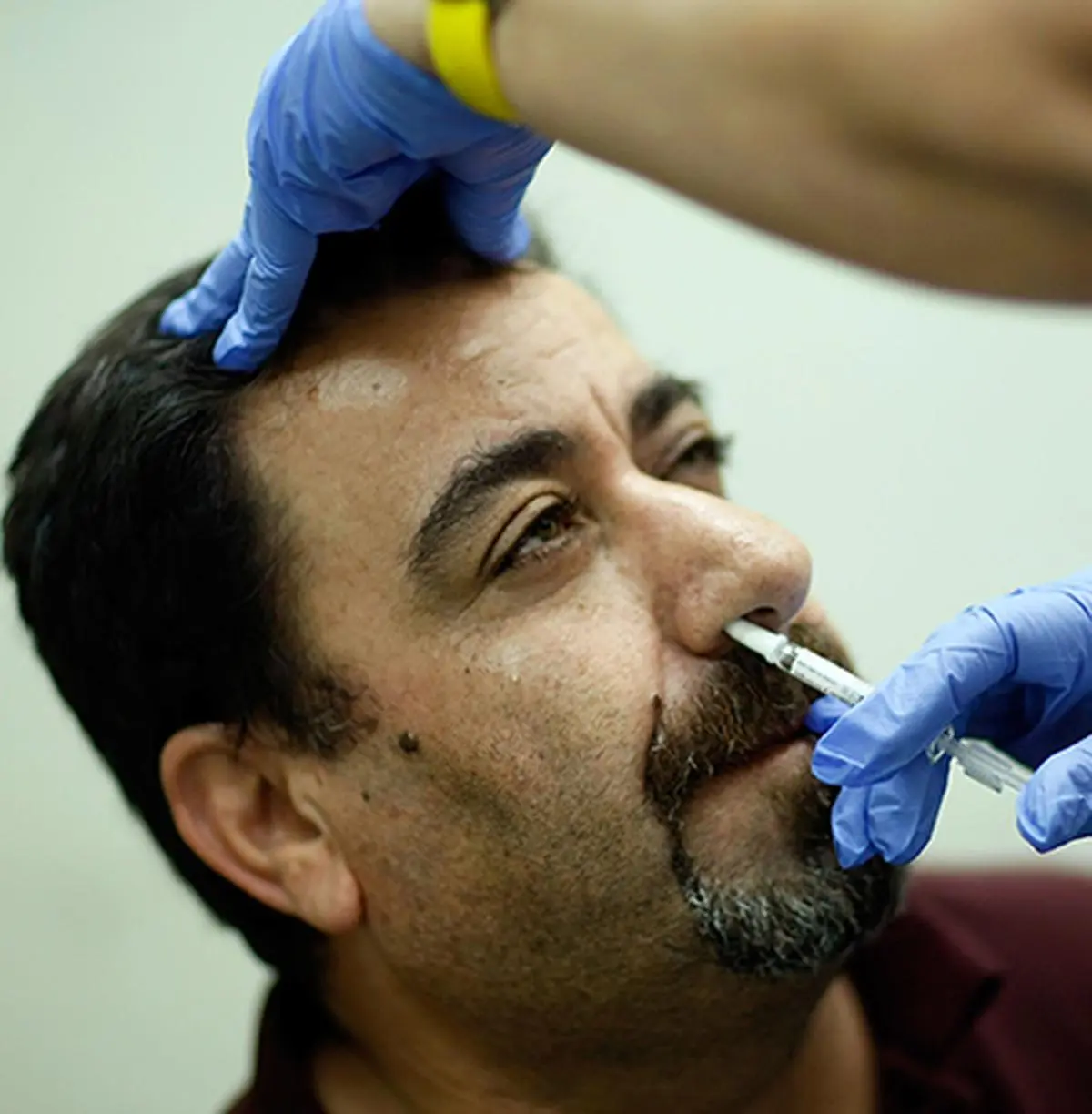

The Central Drugs Standard Control Organisation (CDSCO) has granted emergency use authorisation (EUA) to the booster dose of Bharat Biotech’s Covid-19 nasal vaccine – iNCOVACC, sources said. iNCOVACC is India’s first nasal vaccine developed against Covid-19.
The national drug regulator had given a green signal to the vaccine for emergency use in adults, as a primary dose (it can be administered only to the unimmunised) in September. According to officials, the EUA has been granted (for the third dose), for adults only, irrespective of whether they have been administered Covaxin or Covishield.
The nasal vaccine can be taken six months after the second dose. Its administration will begin post-alignment with the CoWIN application.
Bharat Biotech developed the nasal vaccine in partnership with Washington University-St Louis. While the US university developed the vector that carries the spike protein and evaluated it in pre-clinical studies, Bharat Biotech took care of the product development and manufacturing part. Product development and clinical trials were funded in part by the Centre’s Department of Biotechnology’s Covid Suraksha programme.
The company, in its fact-sheet mentioned, iNCOVACC is an intranasal vaccine that “stimulated a broad immune response”, neutralising IgG, mucosal IgA, and T cell responses. Being an intranasal vaccine, it may produce local antibodies in the upper respiratory tract, which may provide the potential to reduce infection and transmission of Covid-19.
iNCOVACC is stable at 2-8°C. Bharat Biotech had earlier said its manufacturing capabilities at Gujarat, Karnataka, Maharashtra and Telangana would be utilised for production of the vaccination.
SHARE
- Copy link
- Telegram
Published on November 25, 2022
el.parentNode == parent) if (filtered_divs[1]) { var newElement = document.createElement(‘div’); newElement.id = slotName; newElement.setAttribute(“class”, “dfp-ad element no-border hide-system”); filtered_divs[1].parentNode.insertBefore(newElement, filtered_divs[1].nextSibling) } } catch (err) { console.log(“ad error”) }googletag.cmd.push(function() { googletag.display(‘div-gpt-ad-1660731707267-0’); }); }}); ]]>
el.parentNode == parent) if (filtered_divs[4]) { var newElement = document.createElement(‘div’); newElement.id = slotName; newElement.setAttribute(“class”, “dfp-ad element bill-board-ad no-border py-2 mb-3 hide-mobile text-center”); filtered_divs[4].parentNode.insertBefore(newElement, filtered_divs[4].nextSibling) } } catch (err) { console.log(“ad error”) }googletag.cmd.push(function() { googletag.display(‘div-gpt-ad-1660731171391-0’); }); }}); ]]>
el.parentNode == parent) if (filtered_divs[4]) { var newElement = document.createElement(‘div’); newElement.id = slotName; newElement.setAttribute(“class”, “dfp-ad element no-border hide-system text-center”); filtered_divs[4].parentNode.insertBefore(newElement, filtered_divs[4].nextSibling) } } catch (err) { console.log(“ad error”) }googletag.cmd.push(function() { googletag.display(‘div-gpt-ad-1660731946557-0’); }); }}); ]]>